(a)The Royal Institution of Great Britain

Figure 3: Diffusion Coefficients for the different loadings
simulated and trajectory
plot (100 ps) showing preferential diffusion path through 12 MR channels.
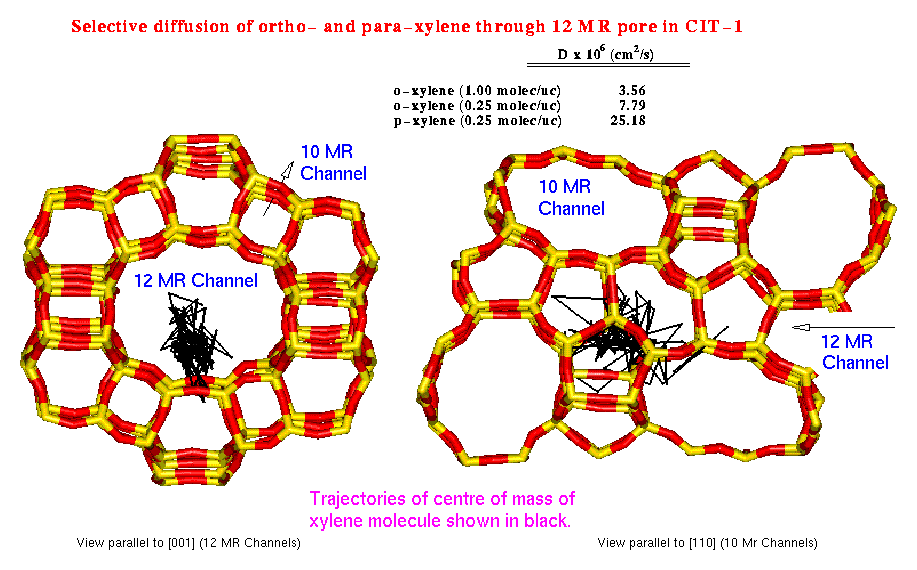
Diffusion of organic molecules in zeolites is an important step to understand reactivity. Diffusivity depends to a large extent on the relative sizes of the zeolite channel and the sorbate molecule; and host-guest interactions can be succesfully treated using an atomistic approach. CIT-1 is a zeolite recently synthesised with a rather unusual channel structure formed by 10 and 12 MR (membered rings). Para-xylene diffuses through 10 and 12 MR zeolitic channels, while the ortho- isomer -being slightly bigger- can only diffuse through 12 MR channels. Diffusion coefficients -difficult to obtain experimentally- and diffusion mechanisms have been unveiled using DL_POLY parallel code on the CRAY-T3D at EPCC. Simulations have been carried out for 100ps at 500K using different loadings of ortho- and para-xylene as sorbate. Thirty-two unit cells of all silica CIT-1 formed the host system comprising 2688 atoms. Diffusion of both isomers (ortho- and para-xylene) through the 12 MR channel system was found more favourable; and diffusivity was found higher for the smaller isomer, para-xylene, (due to less repulsion with the channel walls) and higher for lower loadings (due to less sorbate-sorbate interactions), as shown in figure 3.

Figure 4: Activation energies calculated for the diffusional
process of ortho-
and para- xylene through the channels of CIT-1.
Activation energies for the diffusional path of each of the isomers (ortho- and para-xylene) in each of the two channels systems (12 MR and 10 MR) have also been calculated. The results (figure 4) show how para-xylene can diffuse through both channel systems while ortho-xylene -due to its bigger size- can only diffuse through the biggest channel (12 MR). Activation energies obtained for para-xylene suggest that a certain proportion of them can diffuse through the small channel (10 MR) -this proportion depending on the temperature of the system-although most of the molecules prefer diffusion through the big channels where the activation energy is smaller.